|
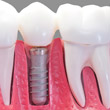
Orthopaedic implantsOrthopaedic implants is a wide term which covers a number of artificial devices which are used to replace joints in the body, e.g. hip, knee, finger and shoulder. Historically the domain of the elderly, orthopaedic implants are now increasingly used across the populace to replace arthritic and/or damaged joints. The combination of the increasing longevity of individuals and the wider use of implants on younger patients, has placed greater demands on manufacturers to produce longer lasting implants. For hip implants, the form characteristics of the femoral cup and head are critical to its load bearing capability. Typically however the main cause of failure in hip joints is due to wear and in particular the particles that are generated. For example the most common cause of femoral bone loss is due to osteolysis. Although the total cause is not known, it has been attributed to a variety of factors including foreign body reaction to particulate debris, in particular to polymeric debris. As such the avoidance of wear in hip joints is critical. This requirement typically leads to a high tolerance on surface finish. Hip joint manufacture is controlled by international standards, which include ISO 7206 (parts I VIII). The requirements for knee implants (and other orthopaedic implants) are similar to hips in that they require good form and surface finish characteristics to ensure they achieve the desired performance standards. Taylor Hobson offer orthopaedic implant manufacturers a range of measurement solutions which assist in improving quality and performance.
|
DentalA large amount of research is completed on an annual basis in the area of dental wear. For dental amalgams (fillings), manufacturers are interested in identifying and improving erosion characteristics. To achieve this they complete wear tests in line with ISO standards, e.g. ISO/TS 14569-1 in which they try to mimic the erosion activity in the average mouth. However wear mechanisms involving teeth and dental restorations in the mouth are very complex. In addition, these mechanisms may differ from one individual to another. Standard wear testing of both dental amalgams and toothpastes consists of using a toothbrush and paste. Typically the toothpaste is rubbed over a reference substrate for a certain number of cycles. The erosion caused by this process is then monitored. There are a large number of oral care products available aimed at improving the appearance of teeth, e.g. filling paste, toothpaste, etc. Filling pastes need to be resistive to wear, while toothpastes and whitening products need to limit the wear they complete on teeth. As such dental manufacturers are typically interested in characterising wear on teeth or filling amalgams (pastes). Taylor Hobson offer dental manufacturers and researchers a wide range of measurement solutions for monitoring the wear characteristics of dental pastes and amalgams.
|
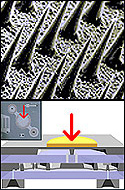
Bio MEMSBioMEMs applies micro devices to biological and medical problems. In their simplest form, technologies in the BioMEMS arena leverage advances in microfabrication and micromachining to create faster, cheaper, hands-off micro and nano scale laboratories, i.e. microfluidics. In more sophisticated forms, BioMEMS devices offer an avenue to artificial organs, personalised drug therapies, and new ways to view cell communication. BioMEMS can be categorised into two categories, Biomedical MEMS and biotechnology MEMS. Biomedical MEMS deal in vivo with the body and the host anatomy, examples of which would include, biotelemetry, drug delivery, biosensors and other physical sensors. Biotechnology MEMS deal in vitro with the biological samples from the host, examples of which would include, gene sequencing, functional genomics, drug discover, pharmacogenomics, diagnostics and pathogen detection/ID. There is currently a large amount of BioMEMs work in the arena of drug delivery systems, using microfabrication techniques. Two main categories are envisioned: micromachined nanopore membranes and microparticles. Nanopore membranes are produced using photolithography, thin film depositions and selective etching to create membranes composed of silicon with highly uniform pores in the nanometre range. Microparticles, unlike conventional particulate drug delivery systems such as polymer microspheres, can be thin planar discs with a thickness and diameter of 1micron. On top of this there are also interesting developments in microneedles, sensors and micropumps, which are all geared at delivering drugs in a rapid and targeted fashion. As the BioMEMS arena expands, the requirement for nanometric metrology increases, which is a area in which Taylor Hobson is very familiar.
|
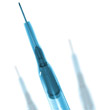
Surgical needlesStandard surgical needles used in the medical arena need excellent quality to ensure efficient performance. They are typically used to transmit or withdraw fluid from the body and as such they need to exhibit excellent flow characteristics. To achieve this they need good internal finish and roundness and cylindricity characteristics. Taylor Hobson have a wide range of experience in addressing the particular requirements of surgical needle manufacturers, which typically entails using special stylii to access the narrow diameters of surgical needles. There is a large amount of work in the field of microneedles, which are being used in a variety of medical areas, including biopsy and drug delivery. Arrays of hollow microneedles can be used to continuously carry drugs into the body using a simple diffusion or pump system. Hollow microneedles could be used to remove fluid from the body for analysis. Microneedles are opening up new areas of medical opportunity like highly targeted drug administration to individual cells, they are also opening a wide variety of metrology challenges which Taylor Hobson are equipped to handle. The challenges include 3D geometric analysis at the micron and nanometric level.
|
Bio chipsBiochips encompass a large area, which includes the field of microfluidics, microchips and lab on chip technologies. They can be broadly defined as measurement devices, prepared using microlithographic or microarraying technologies, that incorporate a biological recognition component. Biochips share much in common with biosensors but are distinguished from biosensors by the use of microlithography fabrication techniques in their production, hence chip. Tremendous interest in microfabricated fluidic channel structures (biochips) has grown over the past decade due to the large number of powerful demonstrations that have appeared in the literature. The diversity of chemical and biochemical measurement techniques implemented on biochips is large including various electrophoretic and chromatographic separations, chemical and enzymatic reactions, noncovalent recognition interactions, sample concentration enhancement, and cellular manipulations. These devices have low cost and small footprints while consuming miniscule quantities of reagents and producing rapid results. Moreover, the manufacturing strategy used to make these devices, i.e., photolithography, allows highly parallel systems to be fabricated at low incremental cost. Biochips open up a vast potential in the area of chemical analysis, however they rely on good fabrication techniques to ensure effective performance. Biochips require good channel structure (height and width) and surface topography to ensure efficient flow of chemicals (bioagents) within them. Taylor Hobson offer biochip manufacturers a range of measurement solutions which assist in improving quality and performance.
|
Cosmetic / dermatologySkin is made up of two kinds of tissue. The epidermis is made up of stratified squamous epithelium and is full of keratin and hardened. The dermis underlies the epidermis, epi meaning above. Wrinkles consist of retractions of the upper skin layer, which are limited by a bulge in a linear way. As the population across the globe ages, so to does the demand for anti aging creams. There is a requirement to verify the performance of these creams but there are also interesting developments within the creams themselves, which include the use of nanoparticles. Cosmetic manufacturers spend vast amounts of money in R&D on new cosmetic products, which are aimed at improving appearance. Along with safety testing, cosmetic manufacturers need to provide quantitative data on the performance of these cosmetic creams and lotions. Although microscopes can provide visual information they are often insufficient for the detailed analysis required. Typically anti ageing cream analysis amounts to analysing a skin sample before and after the application of the cream. Usually replicas of the surface are used, as it is not feasible to measure the actual substrates involved. The Talysurf CLI or CCI platforms with a high-resolution gauge offer an ideal data acquisition tool for this application. While Talymap Textured Surfaces software offers ideal visualisation and analysis. Included in this software is the ability to analyse furrow depth and density along with overall texture direction.
|
PharmaceuticalSolid dose production typically starts with the formation of the drug into particles which range in size from 0.1 to 101¼m. It is often important to characterise these particles as their size and shape can provide useful information on the manufacturing process. Particle size has also been shown to influence dissolution rate, content uniformity and sedimentation rates. The next stage of the pharmaceutical manufacturing process is often to form the particles into a granule by using a binding agent. The resulting granules are typically in the range of a few millimetres and show improvements in flow properties. It is useful to characterise the roughness of granules to correlate this with the manufacturing processes. The final element in tablet manufacture often entails applying a coating to the granules which can serve a variety of functions, including protecting the drug from air and controlling dissolution behaviour. It is often a requirement in tablet manufacture to correlate the surface characteristics of their coatings to their dissolution rate. There are a wide range of requirements for surface characterisation within the pharmaceutical manufacturing arena. One of the more important is in ensuring that the surface finish of pipes and dies in the process area are of a suitable quality to minimise bacterial contamination. Pipes used in pharmaceutical plants can be made from stainless steel or plastic and they can have a range of surface finishes, from 21¼m to 0.21¼m Rq. The surface finish of these pipes is critical when one considers that a common bacteria cell like pseudomonas aeruginosa, which is rod shape can be approximately 0.3 to 0.81¼m wide and 1 to 1.21¼m microns long. Poor surface finish on these pipes could allow for bacteria to build up and would contaminate the manufacturing process.
|
Heart valvesThe human heart is an efficient muscular pump that has four chambers �� two atria and two ventricles �� each closed off by a one way valve. In the course of a day, the heart contracts and expands on average 100,000 times, pumping approximately 2,000 gallons of blood. By opening and closing in a synchronised manner, the four valves keep the blood flowing in a forward direction. More than 250,000 bioprosthetic heart valves are being implanted annually. The ability to replace a diseased heart valve with a prosthetic one has dramatically reduced the mortality associated with heart-valve disorders. Unfortunately, this shining success story is only one side of the coin. The other is the complication associated with all contemporary heart valve prostheses. For example if the prosthetic valve is made of titanium and/or pyrolytic carbon, then they require lifelong anticoagulation. In spite of decades of experience anticoagulation is still a dangerous treatment. Suboptimal anticoagulation carries the threat of major clot formation, leading either to catastrophic immobilisation of the valve leaflets or to the downstream occlusion of a major blood vessel. Quality control is critical in heart valve components as the consequences of failure are catastrophic. Material properties and geometrical shape are all important in the design and manufacture of heart valves. However surface characterisation of heart valves in both 2D and 3D is also critical as it has a large influence on fluid flow and anticoagulation properties of the valve.
|




 More Information
More Information