 |
|
Application 311
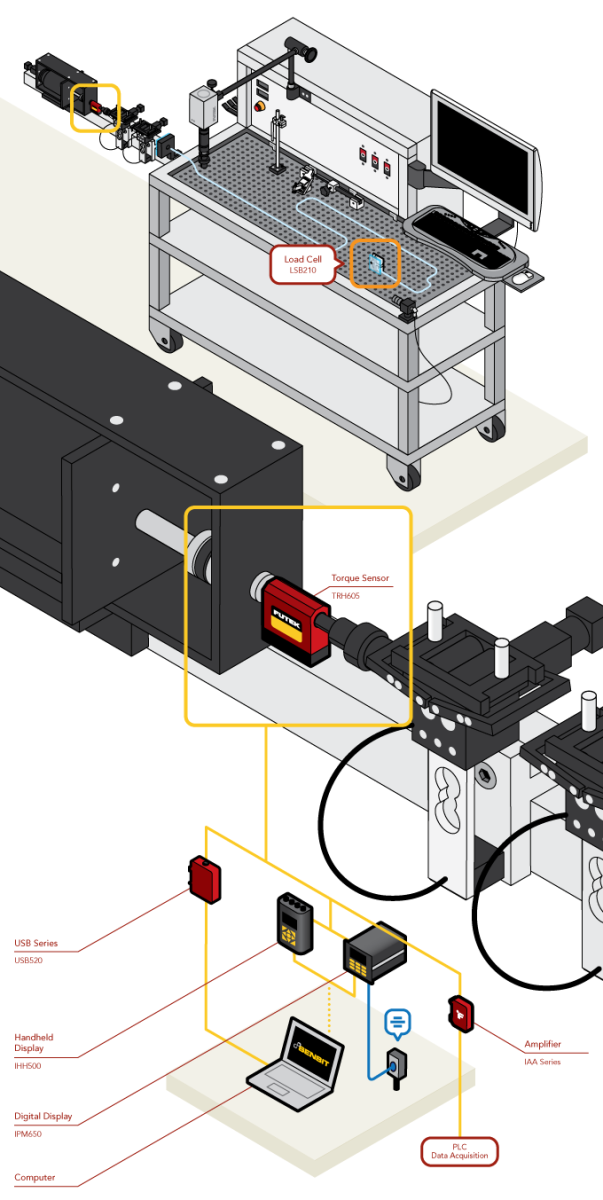
Catheter Torque Test
Application Summary
Interventional device test systems using the ASTM F2394 vascular model are designed to accurately record the performance features of medical including: catheters, guide wires, stent delivery systems, colonoscopes, endoscopes and scope tools. These test systems require precision equipment to satisfy ISO 25539. FUTEK’s Rotary Torque Sensor – Hex-Drive (TRH605) is fixed to the testing system to measure torqueability of a catheter penetrating a tortuous anatomy.
Products in Use
FUTEK’s Rotary Torque Sensor – Hex-Drive (TRH605) paired with USB Solutions and SENSIT™ Test and Measurement Software. |
 |
|
How it Works
|










